We are receiving more and more enquiries from our customers on the subject of hydrogen (H₂). After this topic was dealt with hesitantly at the beginning, it is now picking up speed. The first test networks are in operation and further networks are in preparation. At the beginning, we talked about 10 % to 15 % hydrogen addition. More precisely, a model network with a 30% admixture is also to be operated. This leads to the following questions:
- What effects does this have on the measurement technology in use?
- How do the readings of the devices change?
- Which devices or which operating principles can be used for this?
There are certainly hardly any professionals left who have worked with town gas networks in Germany. The town gas also contained hydrogen with a share of up to 50 % by volume. So hydrogen is not new in the gas distribution. It is a very light, combustible gas with a very wide ignition range and low ignition energy.
Hydrogen (H₂)
- Ignition range in air: 4 – 75 vol.%
- Density: 0.08988 (kg/m³)
- Relative density to air: 0.0695
Methane (CH₄)
- relative density to air: 0.557
(Source: Binas have/vwo (5e editie), informatieboek voor natuurwetenschappen en wiskunde, Verkerk, Noordhoff Uitgevers B.V., 2004)
Hydrogen is characterised by its low density. From a safety point of view, the large ignition range of hydrogen must be taken into account. Even very rich mixtures are still ignitable compared to methane.
How do the different operating principles of the sensors react to hydrogen?
Semiconductor Sensors
With the semiconductor sensor there is almost always a clear reaction to hydrogen. However, the sensors are being produced more and more specifically for individual components such as methane. Therefore it depends on the sensor type used how it reacts to hydrogen.
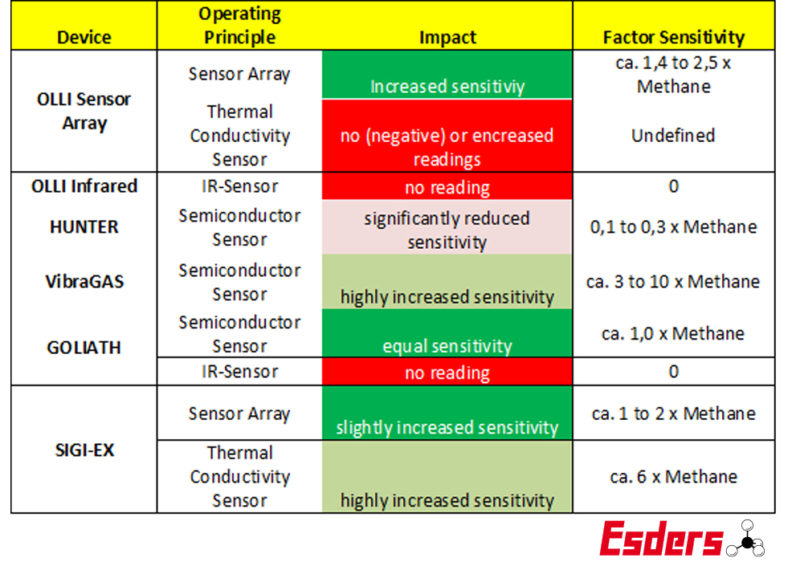
We at Esders also use different semiconductor sensors in our equipment series. While the VibraGAS LS – battery – gas detector and LeckOmiO – rechargeable battery- gas leak detector have significantly increased sensitivity to hydrogen, the semiconductor sensor of the GOLIATH Allround with Ethane analysis is almost identical to methane in its behaviour with hydrogen. And the specialized gas detector HUNTER Gas detection & gas measurement device shows hydrogen in low concentrations with its sensor, but only with significantly reduced sensitivity.
So please contact your equipment supplier about your exact measuring instrument and the sensor used.
Esders Sensor Array
The sensor array evaluates the signals of several sensors and provides a resulting signal for reading. It reacts similarly to methane and hydrogen. It also shows ethane, propane and butane.
Because of the hydrogen, we have hardly any signal loss and still good readings.
This applies to both the and the OLLI.
Infrared Sensor (IR)
The IR sensors cannot detect hydrogen. This means that if a mixture with 20% hydrogen is added, this 20% gas does not cause any indication reaction. Simplified, the reading will be 20% lower than desired. This applies both in the LEL and in the volume % range.
Even hand-held instruments with laser diodes such as the ELLI Esders Laser Leak Indicator Set only indicate methane. Therefore, components such as ethane and propane or butane are not read. This means that, again, reduced proportions in the total gas mixture are available for detection.
Heat Tone Sensor
Heat tone sensors normally indicate hydrogen as well as methane quite well. How much the difference in sensitivity to hydrogen differs from that to methane depends on the sensor type.
Thermal Conductivity Sensor
Thermal conductivity sensors react much more sensitively to hydrogen than to methane. So we are dealing with an over proportional reading. However, there is a very different behaviour of the sensors on hydrogen in larger concentrations. This can go so far that the signal reverses and becomes negative for some sensors in a certain concentration range. It is therefore not guaranteed that every type of thermal conductivity sensor is suitable for the reading of hydrogen in the range between 0 and 100 percent by volume.
The thermal conductiviy sensor of the SIGI-EX reacts uniformly much more sensitive to hydrogen than to methane. Already approximately 20 vol% hydrogen is displayed as 100% methane. For a mixture of natural gas and hydrogen it depends on other components in the natural gas, such as ethane, propane and butane. As gases that are heavier than air, these proportions will weaken the positive signal of hydrogen or will negatively counteract the positive signal.
The thermal conductivity sensor of the OLLI reacts undefined to hydrogen. This means that especially in the range between 4 and 20 vol% hydrogen one cannot say for sure if the sensor detects it reliably.
Below you will find information on the reactions of our device series to hydrogen. Please note that this is the typical reading behaviour, which may vary within a range. We cannot therefore name fixed conversion factors with which a correct measured value could be determined.
Device series and their reaction to hydrogen
Explosion Protection
An equally important question is to what extent the existing equipment is suitable for use with natural gas/hydrogen mixtures with its certification according to the European ATEX directive (explosion protection).
There are no problems for the current networks with admixtures of 10%, 20% and up to 30% hydrogen. Our current certifications include the explosion groups IIA and IIB. The devices are marked with the group IIB. City gas is also included in this group. We can therefore assume that there are no restrictions for these mixtures. The situation is different however for pure hydrogen networks, which are already available for experimental purposes. This requires explosion group IIC, which is currently not available in our measuring devices.
Please note that with this information we want to provide support for the assessment of the reading behavior of our devices and the possible applications.
Please do not hesitate to contact us for further consultation.